Optimizing Cancer Diagnosis to Achieve Better Outcomes
A New Approach
Triopsy’s advanced technology will focus initially on men with prostate cancer but will quickly expand to include patients with all biopsy-accessible sites. More tissue means better accuracy, fewer errors, and lower costs, creating value for physicians and their patients.
Our novel biopsy and pathology technology will aid physicians in assessing cancer size, type, and stage more accurately, while also supporting genomic analysis and pathways to personalized medicine.
Significant and standardized improvement—that’s what motivates us. Our emphasis is on simple, complete, and accurate solutions: optimized biopsy, optimized diagnosis, and … optimized outcome. One patient at a time.
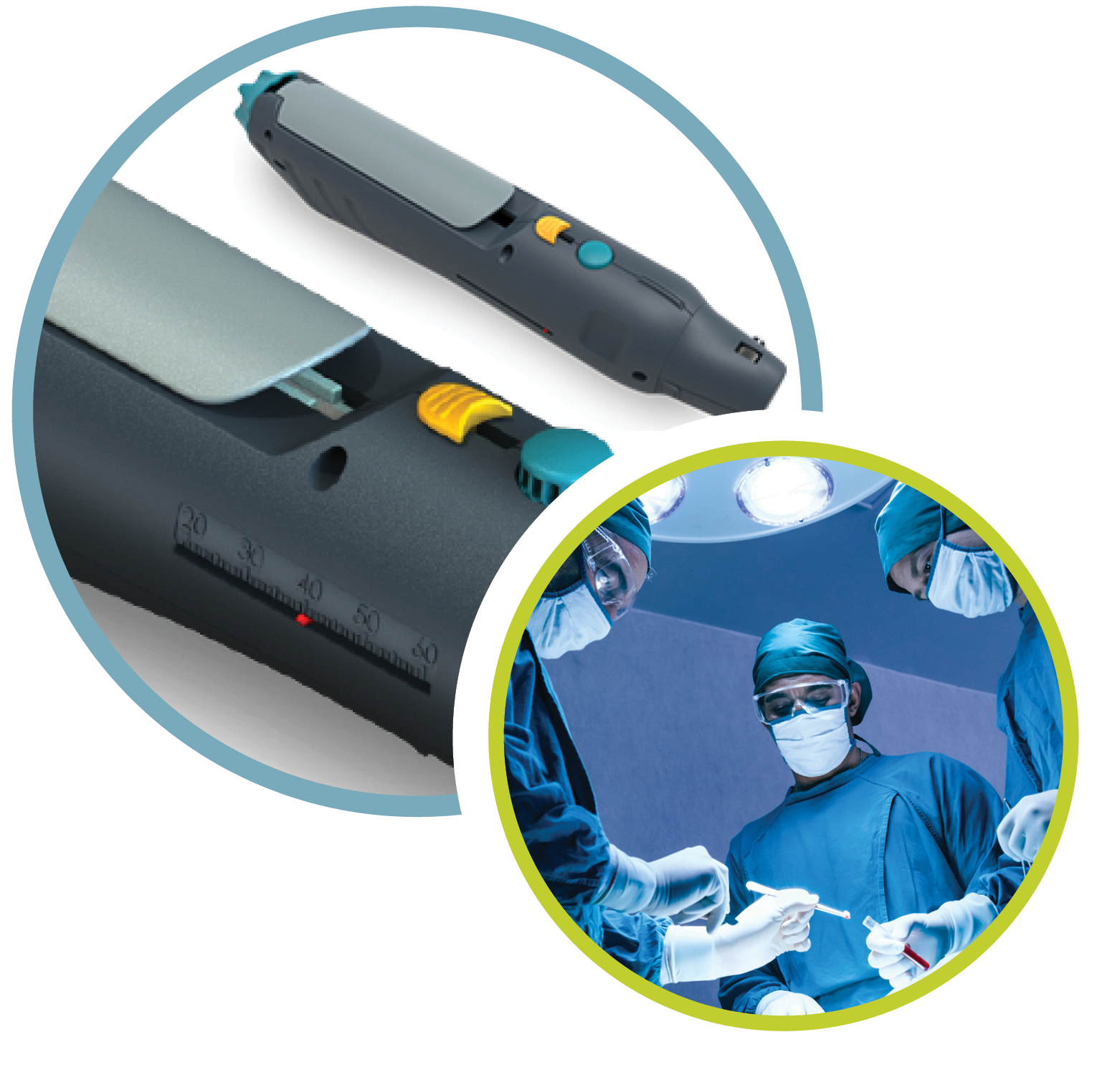
The Triopsy Biopsy System
Needle & Gun
Triopsy’s longer, straighter needle can sample anywhere in the prostate, including the anterior zone and the base, and is equally applicable for both transrectal and transperineal biopsies.
Our device captures up to 6 times more tissue than any other needle. This means that a single Triopsy biopsy can take the place of up to 6 other biopsies. In practical terms, most urologists take 4—5 samples with this longer needle, rather than 12—14 samples with routine needles, creating great time and effort savings.
A series of ridges keep the specimen fully intact and prevent tissue fragmentation, which can cause varying forms of misdiagnosis.
Trocar needle tip reduces needle deflection during penetration of the tissue.
Improves targeting for physicians when using fusion biopsy and image-guided techniques.
Adjustable cannula to take specimens from up to 5.5 cm in length.
The most innovative breakthrough in needle technology in over 20 years; a patented technology.
Specimen Transport
Preserves the orientation of samples during transport and processing.
Prevents specimen handling errors and shortens processing time.
Maintains long, unfragmented specimens.
The specimen carrier platform has a patented Velcro-like surface that effectively “grabs” the intact core sample from the needle bed, keeping it straight and fully intact.
Once sealed, the carriers are placed in a single formalin vial and transported to the lab for processing.
Eliminates the need for contact with the specimen.
Renaissance LIS Software
Our software interfaces with the Tissue Transport System to link specimens to a central cloud-based data server using radiofrequency (RF) or QR code technology, eliminating or greatly reducing transcription errors.
Allows for complete customization of final pathology reports, reducing time spent on developing reports.
Cloud-based Pathology System to track and manage biopsy samples, reporting, and records.
Seamless incorporation of digital images with easy retrieval.
Revolutionizing Patient Care
Our disruptive and innovative technology addresses diagnosing cancer earlier and more accurately. We are targeting a well-defined market opportunity with accessible channels of distribution and pursuing a streamlined 510(k) regulatory pathway with a go-to-market strategy planned for 2024.
Reimbursement codes already exist for our planned products with a reimbursement strategy that benefits all stakeholders—patients, physicians, hospitals, and payers.
We are founded by internationally recognized experts in prostate cancer, an experienced management team, a scientific advisory board, and a board of directors with a track record of success.
Investigational devices. Not available for sale.
Let’s change the world—one sample at a time.
Leadership
BRAD J. BUSCHER
Executive Chairman of the Board
bbuscher@triopsy.com
DAVID G. BOSTWICK MD, MBA, FCAP
Chief Executive Officer
dbostwick@triopsy.com
PAUL ARANGUA, MPH
Director of Research and Product Development
parangua@triopsy.com