TRIOPSY™ BIOPSY SYSTEM
A Seamless System Designed For Accuracy And Efficiency
The Triopsy™ Biopsy System is a fully integrated platform designed to standardize and optimize every step of the biopsy workflow—from tissue capture to pathology—enhancing accuracy, reproducibility, and patient outcomes. Built around four core components that work seamlessly together, Triopsy™ enables earlier, more accurate cancer diagnosis while bringing physicians, radiologists, pathologists, and data scientists together in real time to improve collaboration, efficiency, and patient care.
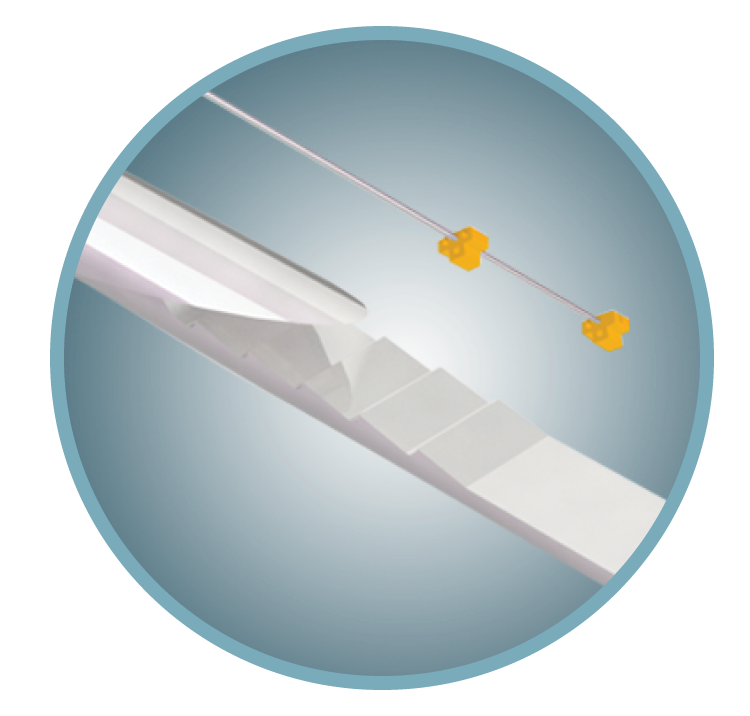
Triopsy™ Needle
High-yield, precise needle for transrectal and transperineal biopsies.
Triopsy™ Actuator
Enables controlled, repeatable biopsy depth for consistent sampling.
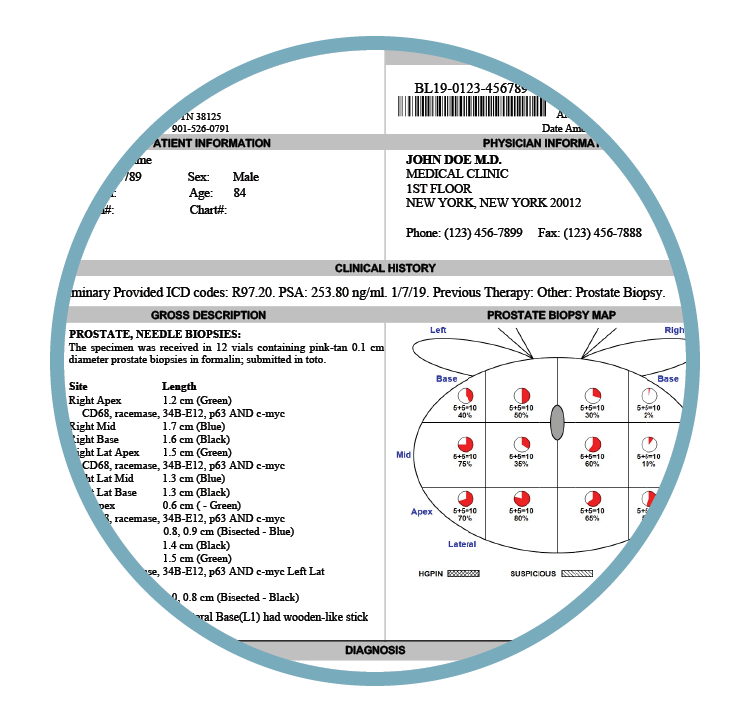
Specimen Transport (IPS™)
Maintains sample orientation and integrity from procedure to lab.